| << return to blog entries
2010-08-16 01:50:07 (5621 views) STAGE 1
First of all, let's admire these beautiful parts.
Just charged in the sun and taken to a dark room:

Another shot:

This is an adjusted version, more representative of the actual brightness the eye sees. It won't help much if your monitor is much brighter or darker than ours...

Some details...

This is what the pigments look like, notice that the two colors on the right don't seem to shine at all and that's because the room wasn't perfectly dark. From left to right: Yellow-Green, Blue-Green (aqua), Purple, Blue, Orange-Red. Blue and Red are the two weakest glowing colors, although red is a color rarely seen in phosphorescent products and it's really neat when you are in total darkness and it's just charged.

Now, you all know the yellow-green color, this is what's been used for the past decade in all manner of toys and emergency signs. However there was a breakthrough a few years ago when a better compound was discovered that glows much brighter and for a longer time. So if you've played with some glow in the dark toy a while ago you may be surprised. When this pigment is fully charged and taken to a dark room it glows as strong as those glow sticks. The benefit of using this color is that it's the brightest and fastest charging (and longest lasting). However it's not so sexy just because it's been overused.

Blue-green (aqua) is just a notch less bright and long lasting than yellow-green, but it's not something you see often, and so we prefer it.

Here's the back view.
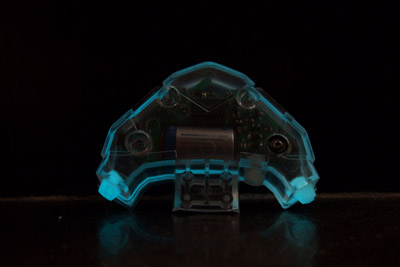
Blue color isn't that far from aqua, but it has very poor performance; it's not nearly as bright and the glow fades very quickly. This is not a winner.

Purple is very hard to capture; this is the original shot:

And this is a color adjusted version that looks closer to what the naked eye sees.

Purple is really awesome, it's a color that is at the far end of the visible spectrum and can't be reproduced very well by your monitor. This pigment looks a lot like the UV lights in a disco. It charges in the sun just fine, however we've had trouble charging it with household lights. We'll try again tonight and post results. All these pigments have very different properties deriving from the quantum-mechanical ways in which photons are stored and reemitted so sometimes they respond well to a narrow range of wavelengths. Sunlight has them all so it's no problem. UV lights should work just fine. Regular lighting... for purple it depends a lot! For aqua and yellow any light is ok. Our theory: the pigments need a charging wavelength that has higher energy than the photons reemitted. So yellow takes anything, but purple needs UV... ?
Now red (orange-red, really, but more red than orange) is hard to explain. This color is totally cool, but it's only bright enough to appreciate in all its beauty for half a minute or so, and you need real darkness to see it - not quite total darkness, but if you've got any sunlight at all coming in it won't look like it's glowing at all.
This is a shot taken after about a minute of charging. The room wasn't perfectly dark.

This is an adjusted version, showing what the eye really sees (actually not exactly, there's a very low wavelength red component here that the camera doesn't capture, these unusual wavelengths (deep red and deep purple), which the monitor doesn't reproduce, are the ones that look coolest because the eye isn't exposed to their hues very often).

Please note that if we wanted to make this look cool in the photos we could have taken the pictures 1 second after charging and they would all look like neon lights. However the purpose here is to achieve some degree of realism...
Here is a brightness comparison. Red vs Purple after 20 seconds of charging, room not perfectly dark.
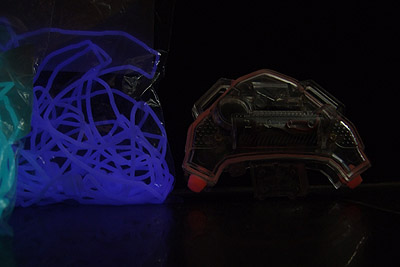
So what's the winner? We like aqua a lot because it glows with some strength and it's not the usual yellow-green. Both yellow green and blue green (Aqua) glow strong enough that the little grommet illuminates surfaces it rests on. Purple does that to some extent too but for a shorter time.

Of course if you happen to be in a club this thing should just glow like crazy because UV is what it likes to eat for breakfast... But we haven't tried a bright UV light from a reasonable distance because... we don't have one. Instead, this is what a tiny purple led does when it's pushed right into the grommet. The light from this LED is not bright at all because it emits only purple and some UV. All the glow you see is from reemitted energy.

If the enclosure is to be clear, the war is on between purple and aqua. We need to test at night, and with different lights especially a real UV light. It's hard to monitor glow decay during the day because the parts look like they're dead very quickly but they aren't really.
The final consideration is the color these parts have in normal lighting (when you can't see them glowing at all).
Purple is pure white in normal lighting, which makes it the best looking. All other colors look off-white (slightly yellowish), except for blue which is almost pure white (but doesn't glow much at all) and red (which looks pale orange).
STAGE 2
We tried the pink transparent enclosure with the purple silicone parts:

Not much difference in the glow color.
Then we remembered that we have some spray painted enclosures on hand.
Black looks mean:


White looks very futuristic with purple:

And even better with aqua:


But as far as futurism goes the winner here is clearly the silver spray painted enclosure with purple glow in the dark silicone:



*This* is something that an advanced civilization would pass down to help train Earth males!
Stage 2 pictures were taken under favorable lighting conditions (light enough to see the enclosure color, dark enough to see the pigments). They show glow levels very soon (seconds) after charging in sunlight for a few seconds (on a not particularly bright day).
Opinions welcome!
Oh, last one: Tron style...

Comments
Post new comment
 |




Don't get me wrong, it looks awesome. I just fear that it will increase the over all price for a feature that may not see much use.